Electrolytic Hydrogen: The Key to Green Ammonia Production
In our first blog, we introduced the concept of Green Ammonia, a sustainable alternative to traditional
ammonia that can reduce carbon emissions across multiple industries. A crucial part of the green
ammonia process involves generating green hydrogen—hydrogen produced using renewable energy
sources. This blog will take a closer look at how electrolytic hydrogen is produced, its significance in
the green ammonia process, and its broader applications in the energy transition.
What is Electrolytic Hydrogen?
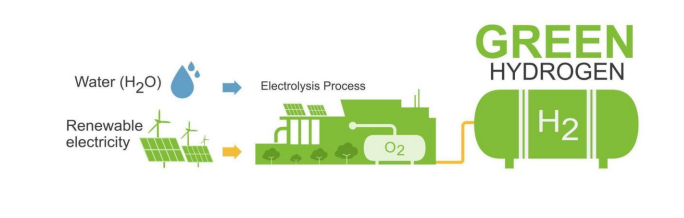
Electrolytic hydrogen refers to hydrogen that is produced by splitting water (H₂O) into hydrogen (H₂)
and oxygen (O₂) using an electrical process called Electrolysis. When this process is powered by
renewable energy (such as wind or solar), the hydrogen produced is carbon-free, making it “green”
hydrogen.
In the context of green ammonia production, this hydrogen is combined with nitrogen (from the air)
in the Haber-Bosch processto form ammonia. The key benefit of electrolytic hydrogen is that it allows
the production of ammonia without relying on fossil fuels, drastically reducing the carbon footprint
of industries such as agriculture and energy
The Process of Electrolysis
Electrolysis is a straightforward process that involves using electricity to break water molecules into
their basic components—hydrogen and oxygen. Here’s how it works:
- Renewable Energy Input: The first step is generating electricity from renewable energy sources,
like wind turbines or solar panels. This clean energy powers the electrolyzer. - Water Splitting: Inside the electrolyzer, water is split into hydrogen and oxygen. This reaction
occurs when electricity is passed through water, resulting in hydrogen at the cathode and oxygen at
the anode. - Green Hydrogen: The hydrogen produced in this process is stored or used immediately for
industrial purposes, such as ammonia production. This is where electrolytic hydrogen becomes crucial
for making the entire process carbon-neutral.
The real innovation lies in using zero-emission electricity to power this process. Since no carbon
dioxide is emitted during electrolysis, it is seen as a cornerstone of future decarbonization efforts
Types of Electrolyzers
There are three main types of electrolyzers used in hydrogen production

- Alkaline Electrolyzers: These are the most commercially mature. They use a liquid alkaline solution
as the electrolyte to conduct ions between the two electrodes. - Proton Exchange Membrane (PEM) Electrolyzers: These use a solid polymer as an electrolyte and
operate at a higher efficiency than alkaline electrolyzers. They are gaining popularity for their
flexibility and rapid response times, making them ideal for renewable energy applications. - Solid Oxide Electrolyzers (SOE): These operate at high temperatures and are still in the research
and development phase. They offer the potential for even higher efficiencies, particularly when paired
with industrial waste heat.
Electrolytic Hydrogen and Its Role in Green Ammonia
Electrolytic hydrogen is essential for green ammonia because it allows the replacement of fossil fuel derived hydrogen with a carbon-free alternative. - Traditionally, hydrogen production for ammonia
involves natural gas in a process called steam methane reforming (SMR), which emits a significant
amount of CO₂. By switching to electrolytic hydrogen powered by renewables, green ammonia
production eliminates these emissions.
Challenges and Opportunities
Although electrolytic hydrogen presents enormous potential, several challenges must be addressed
for its widespread adoption:
- High Costs: Currently, producing hydrogen via electrolysis is more expensive than traditional
methods using fossil fuels. However, with advances in technology and decreasing costs of renewable
energy, this gap is expected to narrow. - Infrastructure Development: The infrastructure required for large-scale hydrogen production,
storage, and distribution is still in the early stages of development, especially for industries that rely
on green hydrogen. - Efficiency: While electrolysis is relatively efficient, improving its energy efficiency remains a key area
of research. Enhancing the efficiency of electrolyzers and scaling up their production will be essential
to making green hydrogen economically viable.
The Future of Electrolytic Hydrogen
Electrolytic hydrogen, when combined with renewable energy, holds the promise of transforming not
only the ammonia industry but also multiple sectors that rely on carbon-intensive processes. As the
global community continues to prioritize net-zero emissions by mid-century, green hydrogen will play
a central role in achieving these climate goals.
Conclusion
Electrolytic hydrogen is a game-changer in the global pursuit of sustainability. By producing hydrogen
in a clean, carbon-free manner, it enables the creation of green ammonia and decarbonizes a wide
range of industries. As technology advances and renewable energy becomes more widely available,
electrolytic hydrogen will help pave the way toward a future with cleaner energy and fewer emissions.
In the next blog, we will dive into the sustainable ammonia synthesis and explore the challenges
and breakthroughs surrounding green ammonia production on an industrial scale.
— Feel free to leave your comments or questions below. Stay tuned for more insights into the future
of green energy
